Abstract
Background: Autologous BMT (either as a planned procedure after completion of initial treatment or as salvage therapy for relapse) is considered standard of care for patients with MM. Therapeutic advances have resulted in significant improvement in survival, necessitating an understanding of the burden of morbidity borne by MM survivors - an understudied topic. We addressed this gap by conducting a comprehensive evaluation of chronic health conditions (CHCs) in MM patients treated with autologous BMT using BMTSS.
Methods: Patients were eligible if they had undergone autologous BMT for MM between 1974 and 2014 at one of 3 BMT centers, had survived for ≥2y after BMT, and were ≥18y of age at participation. Of the 1,116 subjects approached, 630 (56.5%) participated. A nearest-age sibling was invited to participate in the study, and served as an unaffected comparison group (n=289). Survivors and siblings completed a 231-item BMTSS survey that included questions regarding CHCs, including age at onset of each CHC. Each CHC was scored (CTCAE v 4.03) to determine severity. Using multivariable logistic regression, we determined the risk of any severe (grade 3) or life-threatening (grade 4) CHC in survivors compared with siblings, adjusting for age at study, sex, race/ethnicity, education, annual household income and insurance status. Information on initial pre-BMT treatment exposures, conditioning regimens and post-BMT maintenance treatment was abstracted from medical records. Cumulative incidence of CHCs overall, and by specific types were calculated for BMT survivors, treating death as a competing risk. Cox regression was used to determine clinical, demographic and therapeutic predictors of CHCs in BMT survivors.
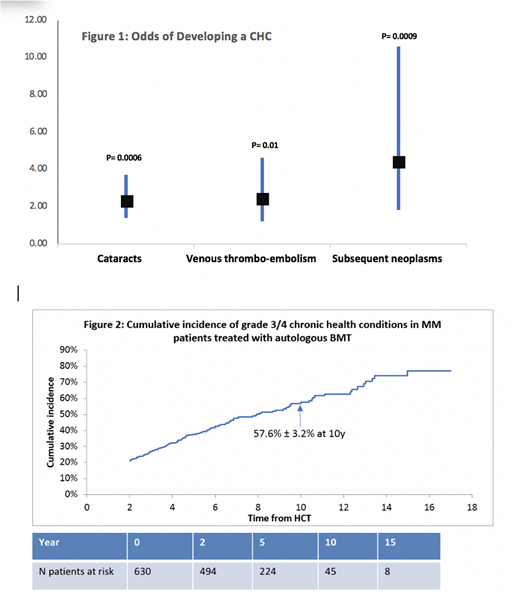
Results: Mean age at BMT was 57.6±8.5y and at survey was 64.2±7.9y. Mean interval between BMT and study participation was 6.6±3.7y; 58% were males, and 62% were non-Hispanic white. Conditioning was melphalan based in 98%, TBI was used in only 5.5%. Mean age at survey for the siblings was 64±8.08y; 58% were male and 84% were non-Hispanic whites. BMT survivors vs. siblings: Overall, 43.3% of the survivors reported a grade 3-4 CHC, placing them at a 1.4-fold higher odds when compared with siblings (95%CI, 1.0-1.9, p=0.03). The odds of developing the following CHCs were significantly higher in BMT survivors when compared with siblings (Fig 1): cataracts (odds ratio [OR]=2.3; 95%CI, 1.4-3.7, p=0.0006), venous thrombo-embolism (VTE: OR=2.4, 95%CI, 1.2-4.6 p=0.01), and subsequent neoplasms (SNs: OR=4.4, 95% CI, 1.8-10.6, p=0.0009). BMT survivors only: 10y cumulative incidence of any grade 3-4 CHC in BMT survivors was 57.6% ± 3.2% (Fig 2). Cataracts: 10y cumulative incidence of cataracts was 24.8% ± 2.7%. Older age at BMT (≥60y: relative risk [RR]=3.3; 95%CI, 2.2-5.1, p <0.0001); TBI-based conditioning (RR=2.3; 95%CI, 1.1-4.8, p=0.02); and female sex (RR=1.6; 95%CI, 1.1-2.4, p=0.01) were associated with increased risk of cataracts. VTE: 10y cumulative incidence of thrombo-embolic events was 10.5% ± 1.6%. Older age at BMT (≥60y: RR=2.2; 95%CI, 1.2-3.9, p=0.007); non-Hispanic white race/ethnicity (RR=4.8; 95%CI, 2.0-11.2, p=0.0003); and pre-BMT exposure to doxorubicin (RR=2.1; 95%CI, 1.04-4.04, p=0.04) were associated with increased risk for VTE. SNs:10y cumulative incidence of SN was 14.0% ± 2.5%. Older age at BMT (≥60y: RR=2.2; 95%CI, 1.2-4.2, p=0.01), pre-BMT exposure to cyclophosphamide (RR=2.9, 95%CI,1.3-6.5, p=0.01) and IMiDs (thalidomide or lenalidomide: RR=3.8, 95%CI, 1.6-9.2, p=0.003); and non-Hispanic white race/ethnicity (RR=2.3; 95%CI, 1.1-4.8, p=0.03) were associated with increased risk for SNs.
Conclusion: The 10y cumulative incidence of a severe/life-threatening chronic health condition approaches 60% in multiple myeloma patients treated with autologous BMT. Cataracts, thrombo-embolic events and subsequent neoplasms constitute the largest burden of morbidity. This study identifies demographic factors and treatment exposures associated with increased risk of chronic health conditions, and provides evidence for close monitoring of these survivors to anticipate and manage morbidity.
Weisdorf:Seattle Genetics: Consultancy; Pharmacyclics: Consultancy; FATE: Consultancy; SL Behring: Consultancy; Equillium: Consultancy. Forman:Mustang Therapeutics: Other: Licensing Agreement, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.